New CRISPR method to enhance CAR-T Cell therapy for cancer
Share :
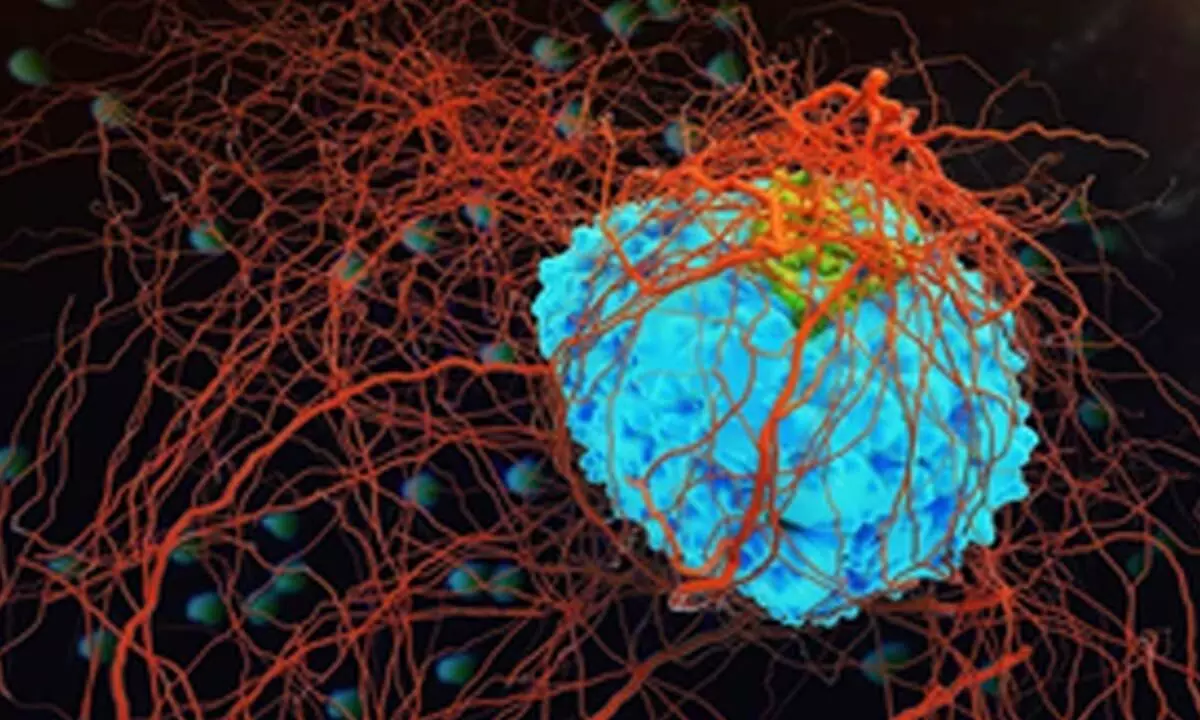
An advanced CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) method to address existing challenges in, and improve, CAR-T cell therapy, targeting both blood and solid tumours, is being developed by German researchers.
New Delhi: An advanced CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) method to address existing challenges in, and improve, CAR-T cell therapy, targeting both blood and solid tumours, is being developed by German researchers.
CRISPR is a distinct technology that allows geneticists and medical researchers to edit parts of the genome by removing, adding, or modifying sections of the DNA sequence.
CAR-T (Chimeric Antigen Receptor T-Cells) cells have shown high efficacy in treating certain blood cancers since their approval in the US in 2017 and in Europe in 2018 for acute lymphoblastic leukaemia (ALL). The therapy involves white blood cells being separated from patients’ blood, genetically modified in the laboratory, and reintroduced as a living drug. A single activated T cell can destroy up to 1,000 tumour cells, ideally remaining in the body for life to eliminate hidden or new tumours.
However, no effective CAR-T cell therapies exist for solid tumours, and remissions are not always durable. The production process for CAR-T cells is also slow and complex.
Dr Karl Petri of University Hospital Wuerzburg is heading the Prime-CAR Inspection project, which utilises CRISPR Prime Editing to enhance cancer-directed immunotherapies. Unlike the conventional CRISPR-Cas9, which introduces double-strand breaks in DNA, CRISPR Prime Editing requires only single-strand breaks, allowing for more precise genome modifications. This method can incorporate all twelve possible base pair substitutions and small insertions and deletions into the T cell genome with high accuracy.
“If CRISPR-Cas9 is described as DNA scissors, Prime Editing is like an eraser and pencil, enabling precise rewriting of DNA," he contended.
His project not only focuses on optimising gene-editing techniques but also aims to standardise safety validation to facilitate clinical translation, ultimately providing more effective CAR-T cell products for patients with multiple myeloma and other cancers.
"Currently, CAR-T cell therapy is approved for selected blood cancers. Our goal is to expand its application to solid tumours and improve its effectiveness for longer-lasting remissions," Dr Petri said.
This research also explores allogeneic CAR-T cells from healthy donors, potentially reducing costs and increasing production efficiency through CRISPR 2.0 editing.







