Indian-origin scientist creates first molecular structure of Omicron protein
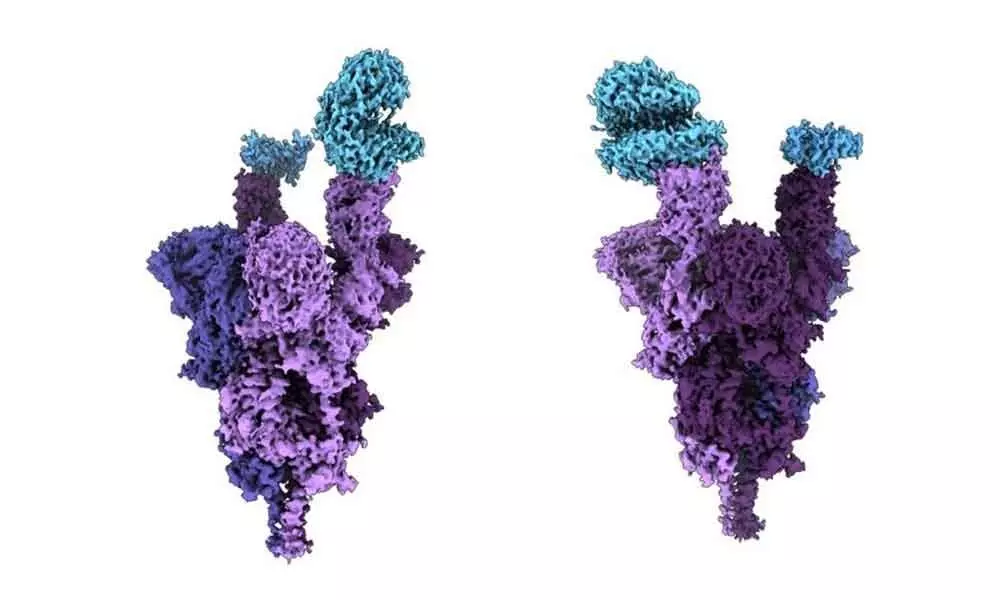
First molecular structure of Omicron protein
An Indian-origin researcher at University of British Columbia (UBC) has created the world's first molecular-level structural analysis of the Omicron variant spike protein.
An Indian-origin researcher at University of British Columbia (UBC) has created the world's first molecular-level structural analysis of the Omicron variant spike protein.
The analysis which is done at near atomic resolution using cryo-electron microscopy, reveals how the heavily mutated Omicron variant attaches to and infects human cells.
"Understanding the molecular structure of the viral spike protein is important as it will allow us to develop more effective treatments against Omicron and related variants in the future," said the study's lead author Dr Sriram Subramaniam, a professor at UBC's department of biochemistry and molecular biology. "By analysing the mechanisms by which the virus infects human cells, we can develop better treatments that disrupt that process and neutralise the virus," Subramaniam added.
The spike protein, which is located on the outside of a coronavirus, enables SARS-CoV-2 to enter human cells.
The Omicron variant has an unprecedented 36 mutations on its spike protein three to five times more than previous variants.
The structural analysis revealed that several mutations create new salt bridges and hydrogen bonds between the spike protein and the human cell receptor known as ACE2.
The new bonds appear to increase binding affinity - how strongly the virus attaches to human cells.
"The findings show that Omicron has greater binding affinity than the original virus, with levels more comparable to what we see with the Delta variant," said Subramaniam.
"It is remarkable that the Omicron variant evolved to retain its ability to bind with human cells despite such extensive mutations."
The Omicron spike protein exhibits increased antibody evasion. In contrast to previous variants, Omicron showed measurable evasion from all six monoclonal antibodies tested, with complete escape from five.
The variant also displayed increased evasion of antibodies collected from vaccinated individuals and unvaccinated Covid-19 patients.
"Notably, Omicron was less evasive of the immunity created by vaccines, compared to immunity from natural infection in unvaccinated patients. This suggests that vaccination remains our best defence," Subramaniam informed.











